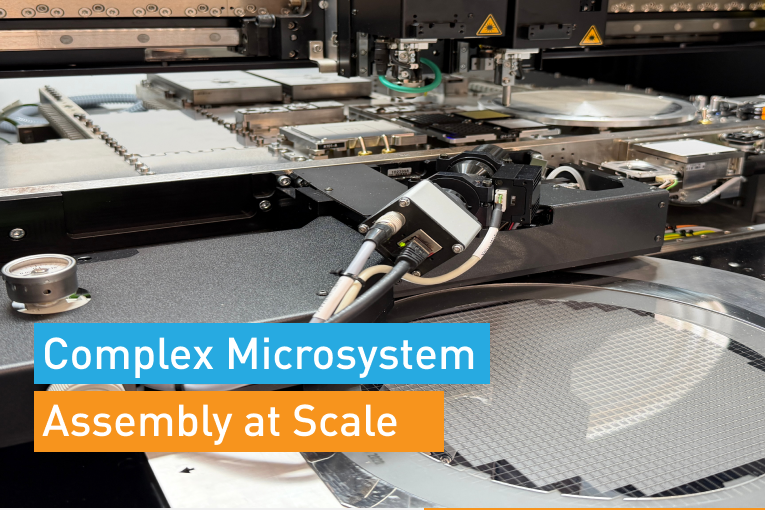
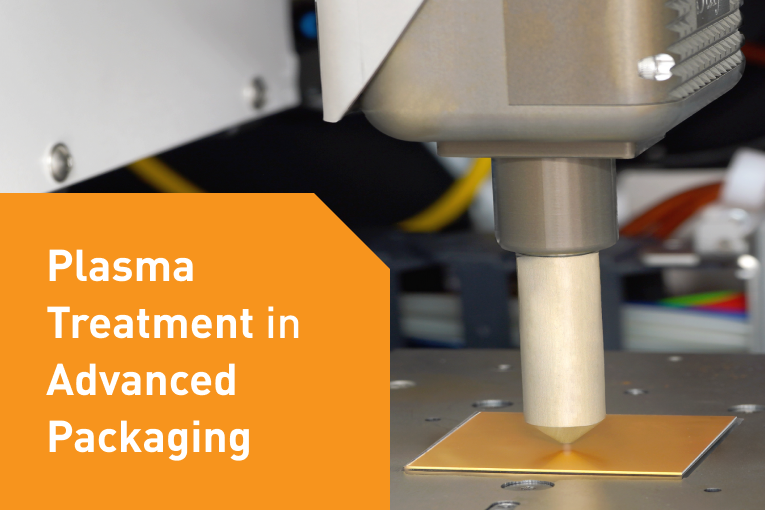
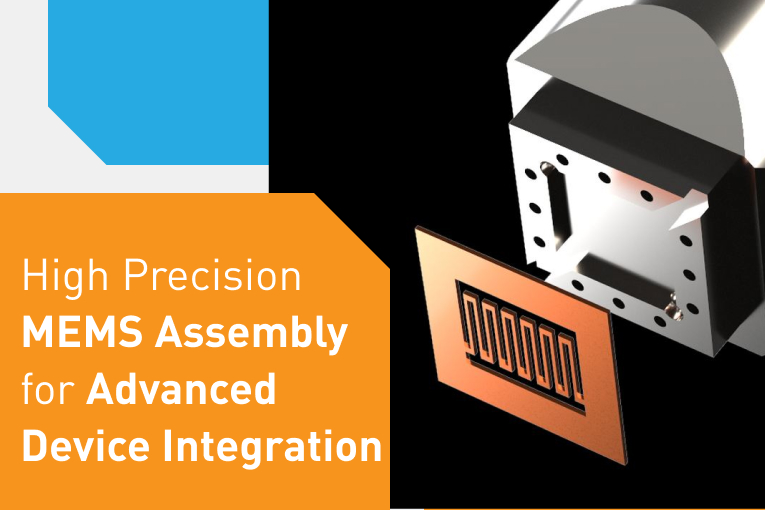
In the high precision world of medical electronics R&D and manufacturing, placement accuracy, process flexibility and process reproducibility are critical.
Here at Finetech, our systems have helped to solve many of the challenges that are associated with developing medical electronics devices and reworking medical components.
Medical companies often approach us with novel applications that push the limits of typical assembly processes. We’ve seen startup companies, founded out of universities, developing new methods of bonding devices together to make a MEMS sensor capable of separating fluids based on viscosity for testing blood pressure, glucose levels and many other critical parameters. We’ve seen national labs looking for a different approach to technologies like sensors, brain probes, artificial retina, 3D ultrasound machines, and many others. We’ve also seen OEMs and contract manufacturers that need a reliable solution to rework an implantable medical device that absolutely cannot tolerate failures, EVER.
The one thing all these technologies have in common is that they present significant bonding and placement challenges. Challenges like precise alignment of complex stacked devices, ultra fine pitch assemblies, and small or unique form factors. In the medical electronics arena, bonding a chip and substrate can mean virtually anything such as; chip to wafer, chip to flex, or flex to flex, and includes processes such as ACF bonding, high force bonding, thermo-compression and UV bonding, and dispensing.
In order to successfully develop these technologies, bonding and rework systems need extreme placement accuracy, process flexibility and controlled parameters. The right system can make the difference between success and failure in cutting-edge new product development. Not everyone has the capability needed to meet these challenges successfully.
I invite you to call us to discuss your die bonding or SMT assembly application.
05/18/2015, created by: Robert Avila